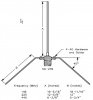
Ok I am at my 3rd attempt at building home brew antennas that consist of a SO-239 and a vertical copper house wire.
I've done this twice before with good results but not now.
For whatever reason I cannot get the solder to stick to the wire or the SO-239.
I am using rosin core solder, copper house wire, fresh SO-239's from Rat Shack and I have tried both a 30 watt soldering pencil as well as an 1100 watt soldering gun. Neither will get the solder to melt to the SO-239. I have even tried flux....still nothing.
I don't know what else to do. What gives?
Below is what I'm trying to build....
I've done this twice before with good results but not now.
For whatever reason I cannot get the solder to stick to the wire or the SO-239.
I am using rosin core solder, copper house wire, fresh SO-239's from Rat Shack and I have tried both a 30 watt soldering pencil as well as an 1100 watt soldering gun. Neither will get the solder to melt to the SO-239. I have even tried flux....still nothing.
I don't know what else to do. What gives?
Below is what I'm trying to build....